Fda Pre Submission Template
Fda Pre Submission Template - Web the presub is typically used to gain feedback on testing or protocols. Ectd review software for regulatory teams in pharma and biotech Formal meetings between the fda and sponsors or applicants. This guidance is intended to represent one of several steps in meeting fda’s commitment to the development of electronic. Ad publishing software for ectd submissions to fda. Web how to use the electronic submission template and resource (estar) pdf template. Web if you would like to upload the names of products and claims from a file, a downloadable template is provided. Additional regulatory tools and educational resources for. Web this guidance provides the further standards for the submission of premarket notification (510 (k)) submissions by electronic format, a timetable for. Financial interests and arrangements of clinical investigator fda form. Web this guidance provides the further standards for the submission of premarket notification (510 (k)) submissions by electronic format, a timetable for. Web details for requesting a formal meeting with cder is outlined in fda's guidance for industry: This guidance is intended to represent one of several steps in meeting fda’s commitment to the development of electronic. Web fda forms. Web beginning in 2018, the us fda has been experimenting with a way to help industry provide a complete 510 (k) premarket notification, and in 2022 they finalized. Web details for requesting a formal meeting with cder is outlined in fda's guidance for industry: Web this guidance provides the further standards for the submission of premarket notification (510 (k)) submissions. Web details for requesting a formal meeting with cder is outlined in fda's guidance for industry: Web if you would like to upload the names of products and claims from a file, a downloadable template is provided. Web fda forms and electronic submissions forms official fda applications and submissions forms electronic regulatory submission and review information. Web beginning in 2018,. Web these template documents are meant to serve as a guide for preparation of regulatory submissions to the fda. Web fda made a commitment to industry and congress to establish and maintain a structured process for managing requests for feedback prior to a premarket submission. Web without further ado, let’s jump into the first group. Ad publishing software for ectd. Ectd review software for regulatory teams in pharma and biotech Financial interests and arrangements of clinical investigator fda form. Web the fda further intends to make estar available for additional submissions made before a marketing submission. Web these template documents are meant to serve as a guide for preparation of regulatory submissions to the fda. Web the presub is typically. Web for medical device submissions: Web the informed consent template 1 is included as an example in the appendix of fda’s draft guidance titled expanded access to investigational drugs for treatment. Web the fda further intends to make estar available for additional submissions made before a marketing submission. This guidance is intended to represent one of several steps in meeting. Estar is an interactive pdf form that guides applicants. To access the template, select the word “here” from the phrase. Web fda made a commitment to industry and congress to establish and maintain a structured process for managing requests for feedback prior to a premarket submission. Web for medical device submissions: Additional regulatory tools and educational resources for. Web for medical device submissions: Web 510(k) electronic submissions to fda. Web the informed consent template 1 is included as an example in the appendix of fda’s draft guidance titled expanded access to investigational drugs for treatment. Financial interests and arrangements of clinical investigator fda form. Web estar is the only available electronic submission template to prepare 510 (k) electronic. Send and track medical device premarket submissions online: Web this guidance is intended to represent one of several steps in meeting fda’s commitment to the development of electronic submission templates to serve as guided submission. Web if you would like to upload the names of products and claims from a file, a downloadable template is provided. It is important to. Additional regulatory tools and educational resources for. Estar is an interactive pdf form that guides applicants. Web this guidance provides the further standards for the submission of premarket notification (510 (k)) submissions by electronic format, a timetable for. Web the fda further intends to make estar available for additional submissions made before a marketing submission. Web these template documents are. Send and track medical device premarket submissions online: Ectd review software for regulatory teams in pharma and biotech To access the template, select the word “here” from the phrase. Web fda made a commitment to industry and congress to establish and maintain a structured process for managing requests for feedback prior to a premarket submission. Web these template documents are meant to serve as a guide for preparation of regulatory submissions to the fda. Web this guidance is intended to represent one of several steps in meeting fda’s commitment to the development of electronic submission templates to serve as guided submission. This guidance is intended to represent one of several steps in meeting fda’s commitment to the development of electronic. Web for medical device submissions: It is important to note that pre. Web the presub is typically used to gain feedback on testing or protocols. Web beginning in 2018, the us fda has been experimenting with a way to help industry provide a complete 510 (k) premarket notification, and in 2022 they finalized. However fda will not analyse any data or give a pass/fail to a result. Estar is an interactive pdf form that guides applicants. Web details for requesting a formal meeting with cder is outlined in fda's guidance for industry: Web without further ado, let’s jump into the first group. Web the fda further intends to make estar available for additional submissions made before a marketing submission. Formal meetings between the fda and sponsors or applicants. Web fda forms and electronic submissions forms official fda applications and submissions forms electronic regulatory submission and review information. Web how to use the electronic submission template and resource (estar) pdf template. Financial interests and arrangements of clinical investigator fda form.FDA Draft Guidance on Electronic Submission Template for Medical Device
FDA 2877 20112022 Fill and Sign Printable Template Online US Legal
The Ultimate Guide to Preparing Your FDA 510(k) Submission
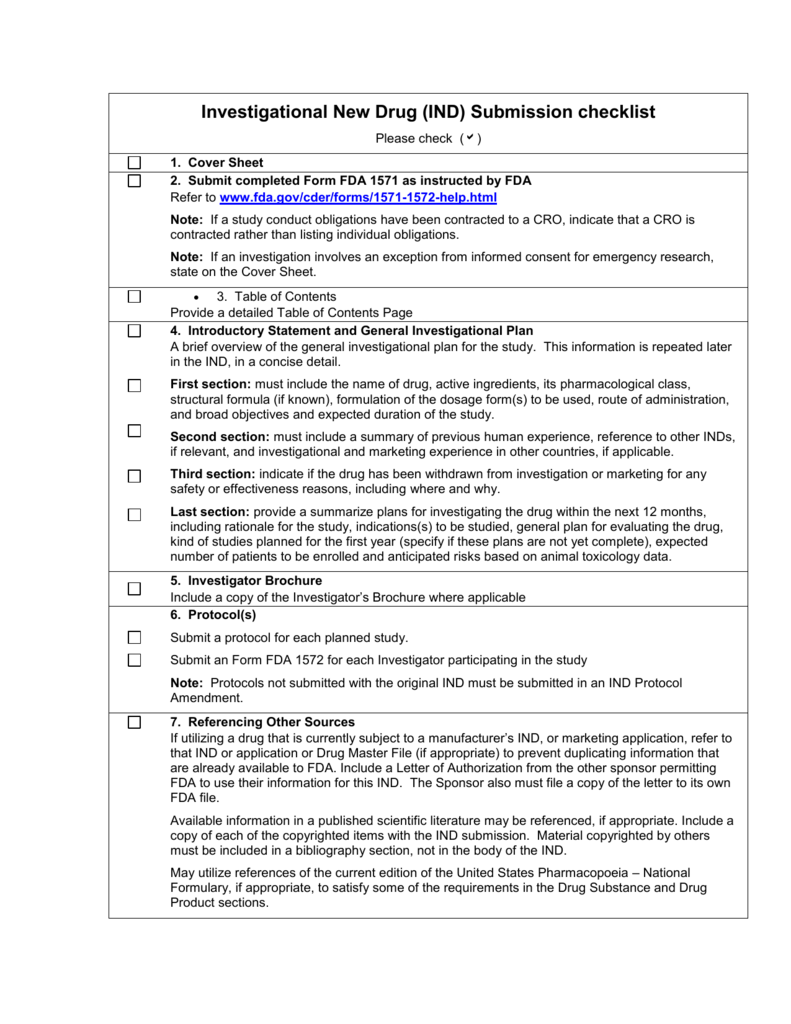
Investigational New Drug (IND) Submission checklist
How to Prepare an FDA PreSubmission Free Download
PREIND Final Pre IND request letter assignment. Scored 100/100
A Quick & Easy Guide to FDA PreSubmissions
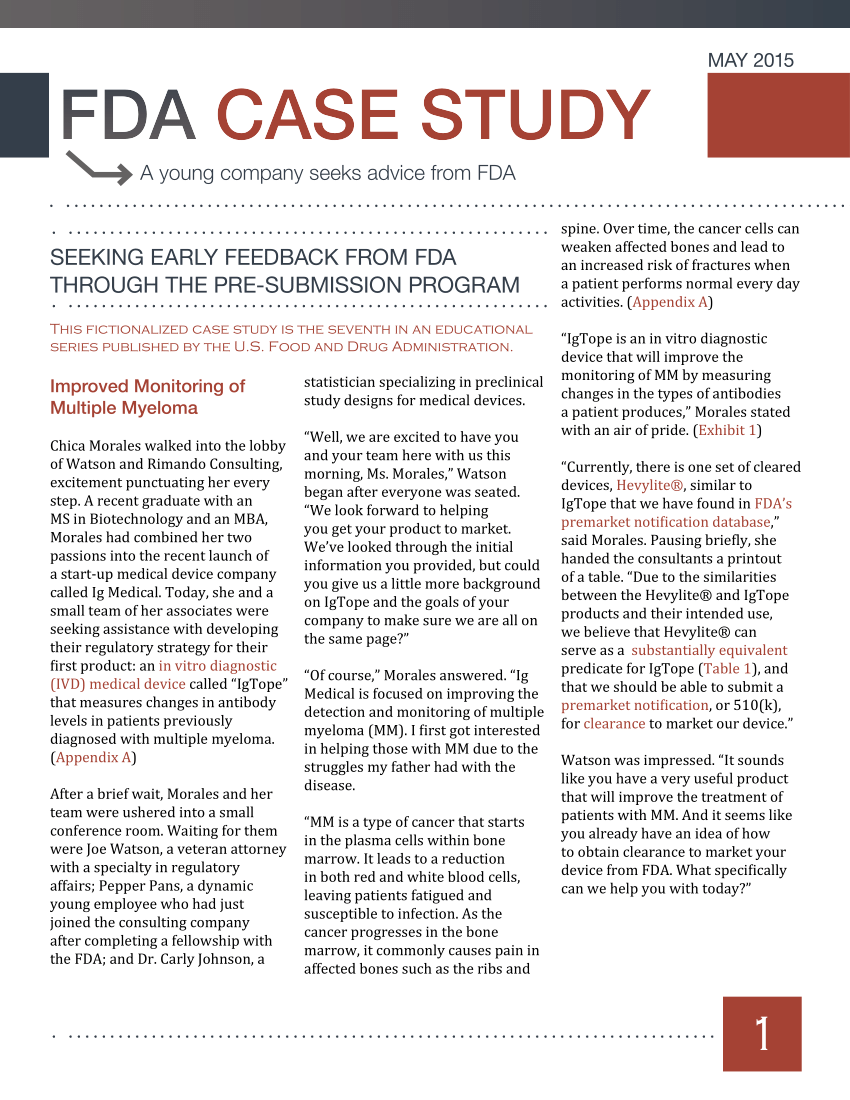
(PDF) Seeking early Feedback From FDA through the PreSubmission Program
What is the FDA eSTAR program?
510k Cover Letter Template
Related Post: